Air pressure and the density of each of the gases in Earth's atmosphere steadily decrease with altitude. At the Earth's surface, the air pressure is on average 1 atmosphere (atm). At 3000 m altitude, the point where most of us begin to have trouble breathing, the air pressure is about 0.7 atm. This means that we have about 70% of the oxygen to breathe as compared to sea level. At 10,000 m, standard cruising altitude for most airliners, the air pressure is about 0.3 atm. This graph shows how air pressure decreases with altitude:
(created by Geek.not.nerd (Wikipedia))
0.3 atm is just enough air pressure to keep most commercial planes flying efficiently. There is not enough oxygen to keep us alive at this altitude. The average air temperature at this altitude is -50°C so oxygen would not be our only problem. Earth's atmosphere is approximately 500 km thick, but most of the gases (90%) are concentrated in the lowermost layer closest to the surface, called the troposphere. There is no exact endpoint to the atmosphere - it just gets thinner and thinner until it disappears into space.
The layers of our atmosphere are visible from outer space, as seen by the space shuttle Endeavour:
This diagram illustrates how the layers of the atmosphere are organized:
(copyright: The Ozone Hole website)
TROPOSPHERE
This starkly beautiful image, taken by the crew on the International Space Station (ISS), shows the lowest level of the atmosphere, the troposphere, to great effect as a deep orange band:
This layer, approximately 17 km deep, ends fairly abruptly at what is called the tropopause. The troposphere is named after the Greek word "tropos" meaning "turning or mixing," an apt name that describes the turbulent activity associated with all the weather that is bound within this layer. Both pressure and temperature decrease steadily with altitude within the troposphere. The composition of this layer is fairly uniform throughout, with the exception of water vapour. Its source is at the surface of the Earth and its concentration decreases very sharply with altitude. Percentage water vapour in the air declines from a maximum concentration of 100% saturation at 100°C, which is approximately 4%, to less than 1% at 0°C. At -50°C, it is virtually zero. Keep in mind that percent water vapour is not the same measurement as percent relative humidity. This graph describes the relationship between water vapour saturation and temperature:
(copyright: GregBenson (en.wikipedia))
There is almost no water vapour in the air at 10,000 m, a good thing because cruising aircraft don't have to worry about the wings continuously icing up. Under special circumstances, however, in-flight wing icing can be very hazardous. When the wings ice up, the ice can alter the wing aerodynamics and reduce their lift force. The plane is then in danger of stalling. When water is very pure it can be super-cooled. This means that even at -40°C, water can exist at liquid droplets inside clouds. It is a rare occurrence that usually happens only in the winter. If a plane flies through these clouds, the water instantly freezes on it because there is now a surface to freeze on. Most jets have pneumatic boots that inflate and break ice off the leading surfaces.
Temperatures in the troposphere decrease from a sea level average of 15°C at the equator to about -55°C and here, where the troposphere layer is thickest, up to 20 km thick, the air at the top of the troposphere can reach -75°C. At the poles, where the troposphere layer is thinner, only 7 km thick, the minimum temperature reaches -45°C. It is also indistinct in the winter when surface temperatures can be as cold as the air 7 km up. This diagram shows this variation in the height of the troposphere:
The polar jet and the subtropical jet (light green letters) are the two main jet streams in Earth's atmosphere. We will explore how they are formed a bit later on in this article. The cells (white letters) are global convective wind currents. The height of the tropopause depends on the average temperature of the entire air mass beneath it, so it is highest at the equator, where the giant Hadley cell circulates air from the warm surface high up into the atmosphere. Exceptionally tall towering thunderclouds tend to breed around the equator fueled by strong moisture-rich Hadley cell updrafts, in a band called the intertropical convergence zone. Here, commercial flights can be challenging, as jets, unable to fly over top of these thunderheads, must instead try to weave around them. The air coming back down at around 30° latitude has little moisture left, and it this is the latitude where most of Earth's deserts exist.
Why does air get colder with altitude? First of all, most of the Sun's energy is absorbed at the Earth's surface, the lowest level of the atmosphere so to speak, so heat is concentrated there. Thermodynamics explains the rest of the answer. When a parcel of air rises, it expands because the pressure it is under decreases. When it expands, it pushes on the air around it, doing work. It doesn't gain heat, however, from its environment because its thermal conductivity is very low (the thermal conductivity is much higher for soil, water etc., than for air and this is why most of the Sun's energy goes right through the atmosphere and heats the surface). Since the parcel of air does work but does not gain heat, it loses net energy and so this is why its temperature decreases. Air temperature decreases at a rate of about 6.5°C for every km in altitude, on average.
Weather
All of Earth's weather is almost entirely confined inside the troposphere (some weather does occur in the next highest layer, the stratosphere, but the mechanisms for how this occurs are not well understood). It is confined here because all weather is driven by density (which depends on temperature and moisture) differences between different pockets of air. Many of these differences are caused by differences in the incident angle of sunlight striking different areas of Earth, so that some latitudes receive more energy input than others. The strong contrast in air temperature between the poles and the equator gives rise to a powerful global wind system called the jet stream. Outside of the tropics, instabilities in the jet stream flow give rise to storms. Within the tropics, weather systems are caused by a variety of different processes, such as seasonal wind reversal in the case of Indian monsoons. Local differences in air temperature, and therefore air density, can be caused by differences in cloud cover and surfaces with different reflectivity or moisture content. These small disparities can merge to produce larger more complex systems, such as fierce thunderstorms. As warm air, carrying moisture, rises through cooler air, it cools causing the moisture within it to condense, releasing energy (in this case latent heat of fusion). This allows the rising pocket of air to cool less than the surrounding air and so it continues to rise further. Through this process, cumulous clouds form, as shown at the far left in the diagram below.
These towering vertical clouds can develop further into cumulonimbus clouds which can contain severe convection currents, resulting in lightning and downpours. Occasionally, these clouds will develop further still into supercells. Areas of organized wind rotation, called mesocyclones, form kilometres up in the sky. Descending rainfall can drag with it an area of rapidly descending air (called a rear flank down draft) that accelerates as it approaches the ground. If it drags the mesocyclone down with it, a tornado forms. The intense low pressure and high wind speeds in these funnels cause water vapour in the air to become visible and this, along with all the debris and dust they may pick up, is why they appear whitish grey like this one:
Weather is a chaotic system in which small changes can grow to have huge effects as a whole. This is what makes weather forecasting difficult.
STRATOSPHERE
The tropopause is an inversion layer. Above this boundary, temperature increases rather than decreases with altitude and very little air mixing occurs, as there is no mechanism to drive convection. Even at this altitude, rare and beautiful clouds, called nacreous clouds, can form. They are mostly visible within two hours after sunset or before dawn, when they blaze with unbelievably bright and slowly shifting iridescent colours, as shown here:
These clouds form well above tropospheric clouds, usually during the winter at northern latitudes. They are so high up that they are fully lit by sunlight while the rest of the sky is becoming dark. The stratosphere generally has no water vapour so clouds cannot form. Occasionally, however, very powerful winds in the troposphere can drive ice crystals far up into this layer. When these crystals come into contact with temperatures of at least -85°C, unusually cold even for the lower stratosphere, these brilliant clouds can form. Deep tropical convective systems can occasionally break through the tropopause as well.
The stratosphere layer lies between the troposphere and the next layer up, the mesosphere. In this photograph, the space shuttle Endeavour straddles the stratosphere and the mesosphere:
The orange troposphere gives way to the white stratosphere and then the bluish haze of the mesosphere.
This layer of atmosphere is stratified into layers of different temperatures. Coldest layers are closest to Earth and warmer layers are farther up. The stratosphere ranges from temperatures typically around -60°C at its lowest altitude to about -3°C near the top. It is usually situated between about 10 km and 50 km altitude. Commercial jets can and often do straddle the lower stratosphere where the low temperatures increase fuel efficiency and low air density decreases drag. Almost all severe weather is safely beneath the aircraft and generally there is no turbulence to deal with. The challenge of stratospheric flight is that air here is of such low density that it does not provide much lift on the wings. As well, jet engines lose power at higher altitudes because there is less air intake into the engines. Finally, commercial jet fuels are only safe above their freezing points. On most flights, pilots descend into warmer air when their fuel temperature warning light goes off. However, on some transpolar flights, it makes more sense to ascend into warmer air. Most operators in Canada use jet fuels with a minimum temperature of -40°C, while some jet fuels used in northern Russia can be used down to -50°C. The new Boeing 787, sporting many technical innovations, will eventually be certified to fly at -55°C. There are many new designs for military stratospheric aircraft and these jets can travel at much higher altitudes than commercial airliners, most of which are light and sport huge wingspans, such as many of those in the U.S. Pentagon's Vulture Project. One prototype, an unmanned aircraft, would have a massive 150 m wingspan, fly at 27 km altitude, and stay up in the air for five years at a time, performing surveillance and communications activities. It faces many technical challenges, such as long-term exposure to extreme solar radiation, the need to minimize weight and the problem of how to power the aircraft. Many designs, this one included, make us of ample solar power at these extreme altitudes and have solar panels installed on the tops of their wings, while others are exploring the use of hydrogen fuel cells.
Stratospheric Ozone
This is the layer that contains the ozone layer, shown here as an azure blue band:
(Image is from netonnet.wordpress, in an excellent article outlining what we can do as individuals to protect the ozone layer)
The stratification of temperature in the stratosphere comes from the absorption of the Sun's energy by ozone gas. In the upper layers, ozone (O3) absorbs UVB and UVC rays (two types of ultraviolet radiation) and splits into molecular (O2) and atomic (O) oxygen gas. This takes place through a reaction called photodissociation. Energy (in the form of UV radiation) is absorbed when ozone bonds are broken to create oxygen ions. Oxygen ions are very reactive and quickly combine with other atoms in the stratosphere. Although these reactions involve both the breaking and making of chemical bonds, they are overall exothermic, which means they release energy. That is why the upper layer, where the majority of these reactions take place, is warmest. The mid layers have less UV radiation passing through them, and less energy is released because fewer of these reactions are taking place. Some heat is released, however, because O2 and O are able to recombine here, an exothermic reaction, and this where most ozone is produced. The lowest layers are coldest because much less photochemical activity takes place there. There is no evidence yet of ozone depletion affecting stratospheric temperatures.
These stratified layers of gases are quite stable because no convective activity occurs here. Horizontal stratospheric circulation does occur however, transporting ozone and other gases. Almost all air enters the stratosphere over the tropics, and most of it moves fairly rapidly east to west around the equator and west to east towards the poles, as this 2-minute simulation shows:
Using these stratospheric conveyor belts, particles such as volcanic dust, may cover the globe in as little as two days. Volcanic ash tends to stay in the troposphere no more than a couple of weeks. Very fine volcanic tephra particles may cover the globe and remain in the stratosphere for a few months and they have only minor effects on the climate. These are generally the particles that contribute to spectacular sunsets associated with volcanic eruptions. The major climate influence from volcanic eruptions comes from gaseous sulphur compounds spewed into the atmosphere, especially sulphur dioxide, which reacts with water in the air to create sulphate aerosols. These very fine particles can stay suspended in the stratosphere for 2-3 years, where they can produce a strong climate cooling effect by reflecting sunlight and modifying clouds as the particles eventually sift down through the atmosphere, contributing to increased cloud cover. This graphic describes the atmospheric effects a typical volcanic eruption:
Looking at this graphic, you may notice that volcanic eruptions are a natural cause of ozone depletion. The ozone layer has naturally replenished itself over the eons through the reactions described above, which tend to maintain an equilibrium concentration of ozone. Chlorofluorocarbons, as well as some other pollutants, overwhelm this natural equilibrium and that is why ozone is being depleted from the stratosphere. Chlorofluorocarbons are discussed in the previous atmosphere article on the composition of the atmosphere. No one is entirely sure how old the ozone layer is, but four billion years ago there was no oxygen, and no ozone, in Earth's atmosphere. When photosynthetic algae began to colonize the oceans, they pumped out oxygen and eventually it rose in the atmosphere. Ozone was created as extreme UV radiation broke oxygen down. Without the ozone layer, deadly UV radiation, especially UVC, would have prevented life from colonizing land on Earth.
Stratospheric Radioactive Fallout
Thousands of atmospheric nuclear tests have been carried out all over the world and there have been extensive studies of both local low level atmospheric radioactive particulates and radioactive dust that travels on the jet streams in the troposphere. This fallout is eventually washed to the surface in rain. Stratospheric radioactive dust, however, can remain airborne for years, and is an even greater concern. As I understand them these tests are not easy to carry out as there are so many variables involved in predicting where this dust may end up. It is much more difficult than predicting long term weather patterns. If you are interested in finding out what we do know about stratospheric radioactive fallout, I recommend this scientific paper released by the U.S. Air Force Institute of Technology in 1987. If you would like to know more about radiation and what it is, I recommend my article, "Radiation: What is Happening In Japan."
Stratospheric Life
Perhaps surprisingly, bacteria survive in the stratosphere, making it part of Earth's biosphere. In 2009, a group of Indian scientists discovered three species of upper stratospheric bacteria not found on Earth's surface and highly resistant to UV radiation. They were collected from a special balloon that took samples of air from different altitudes ranging from 20 km to 41 km. Of course, the scientists had to be meticulous to avoid any possible contamination of terrestrial species, and the 2009 test was a carefully executed repeat of a 2001 experiment, which also found the bacteria.
Occasionally, some species of migrating birds can straddle this zone. Birds have extremely efficient lungs that can extract much more oxygen from the air than our lungs can, giving them a great advantage in high altitude flight. Although most birds tend to fly below 150 metres, many migrating birds, for example, Tundra swans and Whooping swans have been observed flying alongside jetliners cruising at around 7500 m. Bar-headed geese have been observed migrating over the top of Mount Everest, the peak of which stands at almost 9000 m. For humans this is well within what is called the death zone, 8000 m and up. There is only a third of oxygen in the air compared to seal level, and humans cannot acclimatize to this level. Extreme climbers need supplemental tanks of O2.
MESOSPHERE
Unlike the stratosphere, temperatures in the mesosphere decrease with altitude. Temperatures at the upper boundary of the mesosphere, the mesopause, are the coldest temperatures associated with Earth, around -145°C. This layer extends from around 50 km to 100 km in altitude, and like the layers below it, its location is affected by the seasons and by latitude. Temperatures decrease with altitude in this layer because heating by UV absorption of ozone falls off and, more significantly, increasing cooling through carbon dioxide radiative emission occurs. This is how it works: carbon dioxide, like the other greenhouse gases - water vapour, methane and nitrous oxide - is made up of two or more atoms bound loosely enough together to be able to vibrate when they absorb radiation (energy) N2 and O2 gases are too tightly bound to absorb energy this way so they are not greenhouse gases. Eventually these greenhouse molecules emit the radiation again. This absorption-emission-absorption cycle keeps heat near Earth's surface, by reradiating heat in all directions and reducing the heat radiated back out into space. The mesosphere is almost a vacuum, and with so few molecules around to absorb CO2's emissions, there is a net loss of energy as CO2 radiates energy into space.
Clouds, Sprites, Jets and Meteors
And, yet even here in the almost-vacuum of the mesosphere, special clouds, called noctilucent clouds, can form. Their formation requires a temperature of -90°C or lower at an altitude of around 85 km. Like stratospheric nacreous clouds, these clouds are best observed at high latitudes, between 50 and 65°. But unlike nacreous clouds, these are more likely to develop on summer nights. They tend to be bluish-white and rich with undulations. In this photograph, these bright, sharp and eerie clouds glow well after sunset:
(copyright: Martin Koitmae (Wikipedia))
They are made of tiny crystals of water ice and dust. The dust might come from micrometeors or from volcanic eruptions. The water could be formed from the reaction of methane with hydroxyl radicals in the stratosphere, or even the exhaust from the space shuttles. These clouds are rare because water is so rare at this altitude, and what little there is tends to be broken down by UV radiation from the Sun. The mesosphere contains about 1 hundred millionth that of the air moisture in the Sahara desert. These clouds were first known to be observed in 1885, two years after Krakatoa erupted. It is not known if this was a coincidence or not, but more and more of these clouds have been observed since then. Their relatively recent appearance may be linked to climate change, but again, the link is not clear. It is difficult to study this layer of the atmosphere because it is above the maximum altitude for almost all aircraft and below the minimum altitude for orbital spacecraft.
Other mysterious phenomena, such as red sprites and blue jets, occur in the mesosphere as well.
Red sprites, which look like bright reddish-orange flashes, are large-scale electrical discharges that occur high above thunderstorm clouds. This is the first colour image captured of one by NASA aircraft in 1994:
They are often associated with bluish white tendrils hanging below and arcing branches above. Despite often being categorized as such, sprites are not lightning. They are cold plasma phenomena, a bit like a fluorescent tube discharge, that are triggered by lightning in the troposphere below. The physical mechanism responsible for sprite production is still unknown but they seem to be linked to Earth's electrical field system and they may be part of every medium to large thunderstorm.
Blue Jets
Whereas sprites tend to form well above the tops of thunderstorm clouds, blue jets tend to project directly upward from them, usually as a narrow cone. These phenomena tend to form, as a result, lower in the atmosphere, often straddling the stratosphere/mesosphere boundary, as shown in this image which compares them with red sprites and lightning:
(copyright: Abestrobi (Wikipedia))
While sprites seem to be triggered by lightning strikes, blue jets appear to be more strongly associated with intense hail activity. Blue jets are believed to be the result of blue emission lines from neutral or ionized molecular (N2) nitrogen gas. Blue jets are much rarer than red sprites.
Millions of meteors enter Earth's atmosphere every day, with most of them melting or vapourizing altogether as they collide with gas molecules and atoms in the mesosphere. The vast majority of meteors are the size of a pebble or smaller, and their glow as they disintegrate is not even visible from the ground. All these meteor collisions add up to about 50 metric tons of material striking the atmosphere every day, almost all of which evaporates, leaving sparse metal layers such as sodium and potassium in the mesosphere as well as iron oxides and silica-rich nano size particles. These particles, studied using radar data and rocket-borne in situ techniques, are believed to be at extremely low densities in the mesosphere but they may be what nucleates the rare noctilucent clouds that form in this layer.
THERMOSPHERE
The thermosphere is the thickest of all the atmospheric layers, beginning between 80 and 100 km above Earth and extending to between 500 and 1000 km. Its thickness depends on solar activity. For example, it experienced an unusually severe collapse during a recent deep solar minimum in 2008-2009. This layer of atmosphere, the realm of meteors, auroras and satellites, is where solar radiation makes its first contact with Earth. When solar activity is high, UV radiation from the Sun warms the thermosphere, causing it to "puff up like a marshmallow held over a campfire." The opposite happens during low solar activity. The recent contraction, now recovering, was far more intense than the ebb in solar activity can explain, suggesting that we don't yet fully understand all the dynamics of this layer.
Named after the Greek word for heat, the thermosphere can reach temperatures as high as 1500°C during the daytime and when solar activity is at a maximum. A thermometer would not be able to record this heat, however, because the energy lost through thermal radiation would overwhelm the energy transferred from the atmospheric gas. In fact, atoms and molecules are so few and far between in this layer, that there is little to no heat transfer possible between them. This means that even though individual atoms are highly energized in the sunlight, a sensory surface could not "feel" that as heat.
Below the thermosphere, all the atmospheric gases mentioned in the preceding article are mixed together by turbulence, even in the stratosphere and mesosphere where some stratification becomes evident. In this layer, however, different gases tend to form separate layers (with little or no interaction between them) based on their atomic weights. This layer, as a whole, contains mostly molecular oxygen, molecular nitrogen, atomic oxygen, atomic nitrogen and helium gases. At the lowest level, molecular nitrogen and oxygen gases exist in much the usual percentages. Molecules of oxygen and nitrogen are relatively heavier than the other gases, however, and their atmospheric levels tend to fall off as altitude increases. Lighter atomic oxygen and nitrogen gases, and then gases such as hydrogen and helium, become relatively more abundant in the uppermost reaches of the thermosphere. This graphic explains what happens in the thermosphere:
(copyright: John Emmert/NPL from Astrobiologyonline magazine)
As you can see, much of the most energetic, and therefore deadly, radiation from the Sun, such as extreme and far UV radiation, as well as some X-ray radiation, is absorbed and blocked out by the thermosphere. As mentioned, when solar radiation is high, this layer heats up and expands. When it expands it becomes less dense. Although this layer contains on average an extremely low density of gases, changes in density must be taken into account when engineers calculate the orbits of satellites. Higher density contributes to increased drag on these fast-moving bodies and those effects must be offset occasionally by brief boosts from their onboard rockets to keep their orbits from decaying and spiraling down to Earth. The International Space Station (ISS) orbits in the thermosphere at between 320 and 380 km altitude. Here it is, docked with the space shuttle Endeavour, in this May 2011 photograph:
This was the last space shuttle docking with the ISS, and the last mission for the Endeavour.
Although the gases in this layer are stratified, they do circulate thanks to diurnal heating and cooling, creating waves and tides, not unlike ocean tides. Gas ions as well as free electrons and protons, all products of the splitting of gas molecules and atoms by extreme radiation, move along in these tides and collide with neutral gases to produce powerful electrical currents in some parts of the thermosphere. In addition to these currents, and interacting with them, are the aurorae. Aurorae (the Northern and Southern Lights) occur when solar wind, itself made up of highly energized electrons, protons and other ions, collide with ions, atoms and molecules in the thermosphere at high latitudes. The atmospheric atoms and molecules become highly energized as a result and they shed this energy by emitting light at specific wavelengths, depending on the atom or molecule that's energized. For example, excited oxygen molecules emit bright red light and nitrogen molecules emit reddish purple light. The most common colour for aurorae is green and that comes from excited oxygen atoms, as in these Southern Lights captured in 1994:
If you would like to more about how the aurorae work, please see my article called "The Northern Lights."
EXOSPHERE
This is the uppermost layer of Earth's atmosphere, beginning at around 500 km altitude. Its lower boundary with the thermosphere, called the exobase or critical level, is highly variable, depending on how expanded the thermosphere is beneath it. Here there are basically no atomic collisions, because atoms are so far apart from each other. Collisions dominate the motion of gas atoms and molecules beneath this boundary, but above it atoms are governed by ballistic motion, and with sufficient velocity they can and do escape Earth's gravity. It all depends on the velocity and trajectory. Other atoms, with the right trajectory and velocity, orbit Earth a long time, as satellite gases.
The upper boundary of the exosphere extends half the way to the Moon, about 190,000 km. It is defined as the distance where the influence of solar radiation on average atomic hydrogen velocity overcomes the gravitational pull by the Earth. In fact, the exosphere consists almost entirely of neutral hydrogen atoms, the lightest of all the atmospheric gases. Earth's magnetic field protects our atmosphere from being stripped away by solar wind. It acts like an energy collector that interacts with the solar wind material and draws energy out of it. However, Earth's magnetic field also funnels that energy and guides it into the upper atmosphere (this occurs at the poles) and allowing atoms and molecules to escape through the same funnels. There is no cause for alarm, though, because the rate of atmospheric loss through both gravitational escape and escape through the magnetic field is so low, it would take until the Sun becomes a red giant, billions of years from now, to lose appreciable atmosphere.
The exosphere is UV-visible from outer space as a geocorona, which extends to about 100,000 km. This is solar far-ultraviolet light that reflects off of neutral hydrogen atoms in the atmosphere. You cannot see it with the naked eye - Earth's atmosphere appears to end sharply as seen against black outer space:
However, the atmospheric edge looks completely different when seen using UV light instead of visible light. Below is a colour enhancement of an ultraviolet photograph of Earth, with its geocorona extending far out in all directions. Sunlight is shining from the left and the geocorona is brighter on that side. You can make out Earth as outlined by the curvature of the yellow area:
The exosphere plays an important role in the plasma budget of Earth's magnetosphere. It acts as a sink for charged particles, and there is a great influx of them during geomagnetic storms. These charged particles can exchange energy with exospheric neutral hydrogen, allowing them to return to their ground states, removing plasma and restoring the exosphere to its pre-storm state.
IONOSPHERE
Another layer called the ionosphere overlaps part of the mesosphere, thermosphere and exosphere, as shown here:
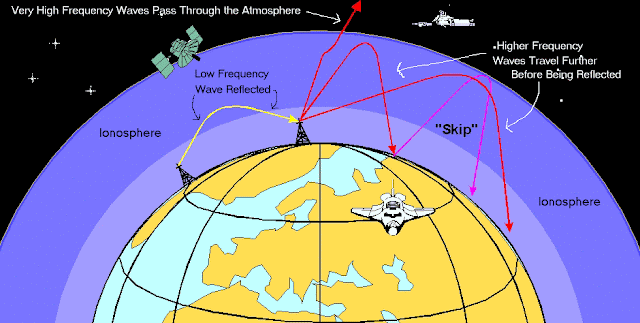
VLF (very low frequency) radio waves can be used to monitor sudden ionic disturbances in the atmosphere. In the daytime, these longer wavelengths dependably bounce off the D layer (image below right). Here, unusual reception patterns can be used to observe the way the ionosphere has been affected by X-ray flares from the Sun. In this way, Sun activity can be monitored. At might, when the D layer disappears, they are reflected by higher E and F layers. When this occurs, VLF wave propagation is strongly affected by ionospheric disturbances, leading to signal variations that are large enough to make monitoring sudden ionic disturbances impossible. This graphic compares daytime and nighttime VLF propagation modes:
(graphic from the SID Monitoring Station website)
The structure of the ionosphere is distributed by gravity waves so the reflective surfaces of ionization can be wavy. This is why ham signals tend to fade in and out and sound fluttery. Gravity waves are an interesting phenomenon. Any fluid, such as water or air, can generate gravity waves when a trigger like an updraft causes a pocket of air to be displaced vertically in stable air. Because of momentum it will overshoot its rise, and then overshoot its subsequent sinking. This motion sets up a series of waves as the air tries to regain equilibrium, as shown here:
This phenomenon also happens with the charged ions of the ionosphere, which also act like a fluid.
During an intense solar flare, hard X-rays from the Sun can reach down into the D layer, greatly increase radio wave absorption and cause radio blackout. Geomagnetic storms can fragment and even temporarily destroy much of the F layer altogether. When this happens, radio transmission becomes sporadic and unpredictable. Ham, ground to air and ship to shore communications are usually affected. During intense geomagnetic storms, communications satellites can be damaged by the influx of plasma higher up in the thermosphere causing disruptions to telephone, TV, GPS and Internet service.
This is the layer of atmosphere that is ionized by solar radiation and forms the inner edge of the magnetosphere. It is a shell of electrons, charged atoms and charged molecules, a ring of plasma in other words, that stretches from about 50 km altitude all the way to the edge of the exosphere, 1000 km. These particles are charged, or ionized, by (mostly) UV radiation from the Sun. In the thermosphere, free electrons can exist for some time before they are captured by a positive ion. It is these electrons that affect radio propagation. And it is this plasma ring that becomes energized during geomagnetic storms. UV, and other kinds of sufficiently energetic radiation can dislodge an electron from a gas atom or molecule, ionizing it. The electron that is released has a great deal of energy and this is the energy that contributes to the high temperatures found in the thermosphere, for example. Free electrons are eventually captured by positive ions, a reverse of the ionization process. At lower levels in the atmosphere, this recombination process prevails because gas molecules, atoms and ions are much closer together. The outer layers tend to remain ionized. The ring current, and Earth's magnetosphere as a whole, forms a complex relationship between Earth's atmosphere and solar activity. This diagram gives you an idea of where the ring current is situated with respect to Earth:
This animation shows how Earth's magnetic field interacts with the solar wind (click on it):
The outermost white lines (magnetic field lines) in this image correspond to the outer capsule (the magnetopause) in the image above this one.
Increased solar wind from a solar storm temporarily compresses the magnetosphere. At this point the thermosphere puffs up and becomes less dense. Eventually the tailward magnetic field lines (all shown as white lines extending outward on the night side of Earth) collapse and Earth's magnetosphere recovers, with the help of the ionization sink of the exosphere.
Now that we are familiar with Earth's atmosphere, we can compare its dynamics with those of other planets, next, in Earth's Atmosphere Part 4.
























No comments:
Post a Comment
Note: Only a member of this blog may post a comment.